BACKGROUND
Insomnia is a widespread disorder that includes difficulty falling asleep, frequent awakenings and difficulty falling back asleep, which causes a person with insomnia to perceive their sleep as too short and of poor quality (American Psychiatric Association, 2013). Cognitive processes associated with hyperarousal as well as stress, climate changes, inappropriate sleep habits, pain, physical illness and mental disorders play an important role in the development of insomnia. Palagini et al. (2016) suggested that sleep-related metacognitive processes (i.e. an individual’s beliefs about the presence of intrusive and disturbing thoughts before falling asleep, which may negatively induce sleep) are related, on the one hand, to arousability as a risk factor for hyperarousability, and on the other hand to cognitive arousal before falling asleep. Buysse et al. (2011) stated that in neurobiological terms, insomnia can be treated as a consequence of dysregulation in the neural sleep-wake mechanisms during NREM sleep. Furthermore, Riemann et al. (2010) suggested that insomnia is the result of an interaction between genetic susceptibility to imbalance in brain mechanisms regulating wakefulness and sleep, psychosocial stressors, and dysfunctional sleep-related behaviours such as worry, rumination, and cognitive hyperactivity at bedtime. Levenson et al. (2015) supported this hypothesis by pointing out that hyperarousal is a state of increased physiological, affective or cognitive arousal that does not allow the subject to temporarily interrupt contact with the environment, which in turn leads to sleep disorders, e.g. difficulty falling asleep. A number of other authors also indicated the importance of a high level of arousal in the development of insomnia symptoms (e.g. Baglioni et al., 2010; Harvey, 2002; Yeh et al., 2015).
Neurobiological concepts of insomnia which emphasize the key role of hyperarousal in the development of insomnia seem close to Pavlov’s hypotheses about the basic properties of the central nervous system (CNS) (Pavlov, 1928). Ivan P. Pavlov, conducting research on conditioning in dogs, formulated the concept of the hypothetical CNS’s basic properties underlying individual differences in the conditioning. According to Pavlov, the CNS includes two independent processes – excitation and inhibition – and is characterized by three constant properties, namely, the strength of the nervous system (the ability of cortical cells to tolerate strong or long-term stimulation without going into the protective inhibition state), mobility (the ability to quickly change from the arousal process to the inhibition process and vice versa, in accordance with changes in the environment) and the balance of nervous processes (the strength of excitation to strength of inhibition ratio). Nebylitsyn (1972), one of the continuators of research on CNS properties, believed that the basic properties of the CNS constitute the basis of human temperament, but understood as stylistic and dynamic features of behaviour, and not the content of behaviour. Furthermore, Bodunov (1993) emphasized that the CNS’s basic properties can be treated as theoretical constructs explaining the inter-individual variability of the dynamics of behaviour, which is expressed in the speed and intensity of the reaction (behaviour), i.e. two main temperament dimensions. According to Strelau (1997), strength of excitation (SE), together with the intensity of environmental stimuli and the state of brain cells, determines the arousal level in the cerebral cortex. In general, genetically determined SE (Strelau, 2008) is a manifestation of arousability, i.e. a feature responsible for individual differences in the brain arousal process. If so, SE might be referred to the neurobiological concepts of insomnia that associate insomnia with hyperarousal processes (Buysse et al., 2011; Riemann et al., 2010). The higher the SE, the more resistant is the nervous system and the lower the level of arousal as a state, which reduces the risk of developing insomnia.
Behavioural manifestations of the CNS’s basic properties in humans are of particular interest. The strength of excitation manifests itself in all the behaviours that testify to the ability of the individual to engage in long-term effort and to operate effectively under load (stress) without turning into protective inhibition, for example lack of responses adequate to the strength of stimulus. In turn, the strength of inhibition (SI) is expressed in behaviour consisting in volitional refraining from any reaction, postponing and stopping activities when necessary. The mobility of nervous processes (MO) is expressed as the ability of an individual to quickly change behaviour in line with changing conditions, as well as to respond quickly and adequately to changes in the environment (Strelau et al., 1995).
The concept of the CNS’s basic properties became the starting point for the development of the psychometric measure of the three features of the nervous system (SE, SI, and MO) known as the Pavlovian Temperament Survey (PTS; Strelau et al., 1999). Using the PTS scales, significant correlations between CNS properties and many other personality (temperament) traits, including those of the Big Five, were revealed. SE correlated positively with briskness, endurance, activity, flexibility, and extraversion; and negatively with fear, distress, perseveration, emotional reactivity and neuroticism (Strelau, 1997). The correlation of SE with the dimensions of personality (temperament) responsible primarily for negative emotions, especially neuroticism, suggests that SE may have similar importance for the development of insomnia (Baglioni et al., 2010; Calkins et al., 2013; Gurtman et al., 2014; Harvey et al., 2014; Jeronimus et al., 2016).
The hypothetical relationship between SE and insomnia may be mediated by arousal-related mood dimensions postulated by Matthews et al. (1990). According to Matthews and co-workers, mood is “an emotion-like experience lasting for at least several minutes” (p. 17). In this model, three correlated mood dimensions were distinguished: energetic arousal (EA), characterised by the contrast between two poles, vigour/energy and fatigue/tiredness; tense arousal (TA), characterised by the contrast between tension/nervousness and relaxation/calmness; and hedonic tone (HT), defining the mood in the dimension of pleasantness and unpleasantness. Both EA and TA dimensions, as well as SE, are arousal-related dimensions important for the relationship between SE and insomnia. Schimmack and Reisenzein (2002) showed that EA and TA could be regarded as two distinct types of activation (arousal) relevant to sleep and insomnia. Only EA and TA were associated with autonomic arousal measures, while HT was not (Matthews et al., 1990). Oniszczenko et al. (2019) showed that EA served as a mediator between affective temperaments and insomnia in the general population. The association between EA and TA and insomnia was also confirmed among male prisoners (Oniszczenko & Romsicka, 2020).
To our knowledge, there has been no previous research connecting Pavlov’s hypothesis on the CNS’s basic properties with insomnia. However, there are theoretical premises to predict such a relationship when the aforementioned role of SE is taken into account. The main goal of our current study is to demonstrate the relationship between the CNS’s basic property, namely SE, and insomnia, and to determine the role of the mood components (EA and TA) as mediators of this relationship. The question therefore remains whether the CNS’s basic properties are related to insomnia and whether the arousal-related mood dimensions may serve as mediators in this relationship. We hypothesized that SE directly and indirectly via arousal-related mood dimensions may be related to insomnia. We expected EA and TA to mediate the relationship between SE and insomnia.
PARTICIPANTS AND PROCEDURE
PARTICIPANTS
The study involved 149 people, 85 women and 64 men, aged 18 to 60 (M = 30.11, SD = 11.43) selected from the general population using snowball sampling. In terms of education levels, 83 participants had received higher education, 59 participants secondary education and 7 participants primary education. Sixty-six participants were single, 78 married and 5 divorced. Twenty participants lived in the countryside, 19 in small towns and 110 in large cities.
PROCEDURE
The study was anonymous and participation was voluntary. The participants did not receive any remuneration for participating in the study. All self-reported questionnaires were administered in a rotated manner. Each participant proceeded to fill in the questionnaires when they agreed with the study rules previously provided. The research procedure was approved by the Research Ethics Commission at the University of Warsaw, Faculty of Psychology (ref. 2-28-02-2018).
MEASURES
Pavlovian Temperament Survey. The CNS’s basic properties were measured using Strelau and Zawadzki’s Pavlovian Temperament Survey (1998). The questionnaire describes the strength of excitation, the strength of inhibition and the mobility of nervous processes in accordance with Pavlov’s concept. It contains 57 items with a 4-point rating scale (fully agree, moderately agree, moderately disagree, strongly disagree). High scores on the scales indicate a high level of the diagnosed features.
UWIST Mood Adjective Checklist. Mood was assessed using the Polish adaptation of the UWIST Mood Adjective Checklist (UMACL; Matthews et al., 1990; Polish adaptation, Goryńska, 2005), a 29-item questionnaire that provides measures of three mood dimensions: hedonic tone, energetic arousal, and tense arousal. High scores on the scales indicate a high level of diagnosed mood dimensions.
Athens Insomnia Scale. To assess insomnia symptoms, the Athens Insomnia Scale (AIS; Soldatos et al., 2000) in its Polish adaptation (Fornal-Pawłowska et al., 2011) was used. The AIS consists of eight items, scoring from 0 to 3. Higher values indicate greater severity of insomnia symptoms.
Cronbach’s α values for all scales in all questionnaires are displayed in Table 1.
Table 1
Descriptive statistics, Cronbach’s α values and Pearson’s r correlations between Pavlovian Temperament Survey, mood and insomnia scales in the sample (n = 149)
STATISTICAL ANALYSIS
Statistical analysis was performed using IBM SPSS Statistics 27. The relationships among variables were examined with Pearson product-moment coefficients. The mediation analyses were conducted via the PROCESS macro for SPSS v. 3.5, model 4 (Hayes, 2018). A bootstrapping procedure with 5,000 sample draws and bias-corrected standard errors was used to estimate the direct and indirect effects (Preacher & Hayes, 2008).
RESULTS
Our study was cross-sectional and correlational with analysis of mediating effects. Data normality was checked based on the skewness and kurtosis values. All the data analysed in the study fulfilled the criterion of compliance with the normal distribution.
Table 1 contains data on descriptive statistics, Cronbach’s alpha values and Pearson’s correlations for the variables studied. As seen in Table 1, SE negatively correlated with insomnia (no significant correlations between SI and MO and insomnia). SE, SI and MO positively correlated with HT and EA, while the correlations between all the CNS’s properties and TA were negative. HT and EA were negatively correlated with insomnia but TA as the mood dimension positively correlated with insomnia.
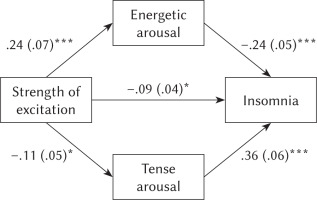
Two separate analyses were performed using a bootstrapping procedure, with EA as the mediator between SE and insomnia and with TA as the mediator between SE and insomnia in the studied group. Our rationale is that SE as a working capacity of nerve cells may affect arousal-related mood dimensions, which then causes insomnia. The first analysis revealed a significant indirect effect of SE on insomnia through EA (effect = –.06, SE = .02, 95% CI [–.10, –.02]). The second analysis found a significant indirect effect of SE on insomnia through TA (effect = –.04, SE = .02, 95% CI [–.08, –.01]). The individual pathways in the mediation analysis are displayed in Figure 1.
Figure 1
Mediating effect of energetic arousal and tense arousal
Note. Mediating effect of energetic arousal in relationship between strength of excitation and insomnia (upper) and mediating effect of tense arousal in relationship between strength of excitation and insomnia (lower) in a studied sample. Unstandardised coefficients are reported, with standard errors in parentheses. *p < .05, ***p < .001.
DISCUSSION
In our study, we intended to show the relationship between strength of excitation as one of the CNS’s basic properties and insomnia, and to determine the role of the mood components as mediators of this relationship. The results indicated a weak negative correlation of SE and insomnia and no correlation between SI or MO and insomnia. The findings are in line with our expectations. This result indicates that people with high SE exhibit fewer symptoms of insomnia.
Strelau (1983), referring to the research by Nebylitsyn (1972), indicated that the SE dimension can be treated as a bipolar property defined as the sensitivity and efficiency of the nervous system cells. A high level of SE is associated with low sensitivity but high efficiency (ability to work) of brain neurons, while a low SE level is associated with high sensitivity and low neuron efficiency. High SE level individuals are characterized by a low level of arousability, making them less susceptible to stimuli that interfere or may interfere with falling asleep. The physiological mechanism of SE is difficult to explain, but it can be hypothesized that we are dealing with a genetically determined property of the brain tissue underlying the mechanism of insomnia. Behavioural genetic studies based on the psychometric measurement of this property showed a high level of heritability of SE in both the Polish and German populations – 83% and 85% respectively (Oniszczenko et al., 2003).
As mentioned before, SE is positively correlated with other dimensions of personality (temperament) based on the concept of arousability, for example extraversion or emotional stability, which suggests the ability of people with high SE levels to cope better with stress conditions (Strelau, 1995, 2001). As Harvey et al. (2014) suggested, stress plays an important role as a predisposing factor for insomnia development.
The results did not confirm the association of SI and MO with the symptoms of insomnia. It seems that SI and MO are irrelevant to insomnia, because their roles are manifested in situations other than falling asleep. For example, SI is important when one needs to refrain from acting out or interrupting certain behaviours in response to the demands of the situation. MO in the behavioural sense, in turn, is associated with the speed and ease of changes in behaviour in accordance with the changes (requirements) of the environment.
The results confirmed that the two arousal-related mood components, EA and TA, are significant mediators in the relationship between SE and insomnia. These results are not surprising, because arousal is the common substrate of SE and the mood components. SE is directly related to the symptoms of insomnia, but it can also indirectly influence insomnia via the changes in EA and TA as arousal-related components of the mood. The negative correlation between EA and TA observed already in previous studies of male prisoners (Oniszczenko & Romsicka, 2020) suggests that a high level of EA resulting, for example, from physical activity reduces the TA level associated with emotional distress, such as worry about sleep among people suffering from insomnia. On the other hand, an increase in TA can reduce the level of physical fatigue. We observed a negative correlation between EA and insomnia, and a positive correlation between TA and insomnia. So it can be concluded that a high level of EA reduces the symptoms of insomnia, while a high level of TA increases the symptoms of insomnia. Thus the TA high level resulting from emotional distress (for example, cognitive activity that adversely affects the state before falling asleep) is of greater importance for insomnia symptoms (Schmidt et al., 2011).
Interestingly, SE correlated positively with EA and negatively with TA and insomnia. This suggests that SE is more related to the energetic aspect of behaviour and has a tonic effect on TA and insomnia symptoms at the same time.
The study we conducted had some limitations. First of all, our result of the mediation analysis should be interpreted carefully. It is worth noting that the statistical confirmation of the mediation model cannot be widely applied when the data come from a one-time correlation study. In such a case, the result may indicate mediating or confounding factors (MacKinnon et al., 2000). The sample participating in the study was small and its selection was not representative. The study was cross-sectional, which makes it difficult to formulate unambiguous conclusions regarding the relationship between the studied variables. The prevalence of anxiety and depression in the study sample, which are significant for the incidence of insomnia, was not screened. Somatic health disorders and their treatment, which may be relevant to the symptoms of insomnia, were not controlled. It would seem justifiable to increase the sample and use the longitudinal research model to verify the obtained results.
Despite its limitations, the study contributes to understanding the importance of SE and the mood components in regulating the insomnia level.
CONCLUSIONS
The results of the study indicate the relationship between SE and insomnia as well as between SE and energetic and tense arousal as mood dimensions related to arousal. Mediation analysis suggests that both energetic and tense arousal may be mediators of the relationship between SE and insomnia. However, the results of the mediation analysis require careful interpretation.


